Science
(www.olympiadsuccess.com)
Chapter 4: Structure of Atom
Class: IX
Exercise number 49A
Question 1
On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer 1
Thomson’s atom model, says that, an atom consists of both negatively charged and positively charged particles which are equal in magnitude and thus, they balance each other’s effect and make an atom neutral.
Question 2
On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Answer 2
Protons (positively-charged particles) are present in the nucleus of an atom.
Question 3
Draw a sketch of Bohr’s model of an atom with three shells.
Answer 3
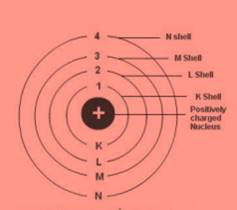
Question 4
What do you think would be the observation if the α-particle scattering experiment is carried out
using a foil of a metal other than gold?
Answer 4
If we use a foil of a metal rather than gold in the α-scattering experiment, then there won't be any change occurs in the observation. Because, in the α-scattering experiment, a gold foil was taken because gold is malleable and a thin foil of gold can be easily made from it and it is difficult to make such foils from other metals.
Yearlong program for Olympiads preparation & to build necessary skills for future.
Explore More
Time to mark your calendar with the upcoming Olympiads exam schedule.
Explore More
Take your Olympiad preparation to next-level by taking LIVE Classes.
Explore More
Assess your performance by taking topic-wise and full length mock tests.
Explore More
Online tuitions for international compeitions like SASMO, SEAMO, etc for Grades 1-11.
Explore More