Science
(www.olympiadsuccess.com)
Chapter 1: Matter in Our Surroundings
Class: IX
Exercise 2
Question 1
The mass per unit volume of a substance is called density (density = mass/volume). Arrange the following in order of increasing density − air, exhaust from chimney, honey, water, chalk, cotton, and iron.
Answer 1
The given substances in the increasing order of their densities,
Air > Exhaust from chimney > Cotton > Water > Honey > Chalk > Iron
Question 2
Answer 2
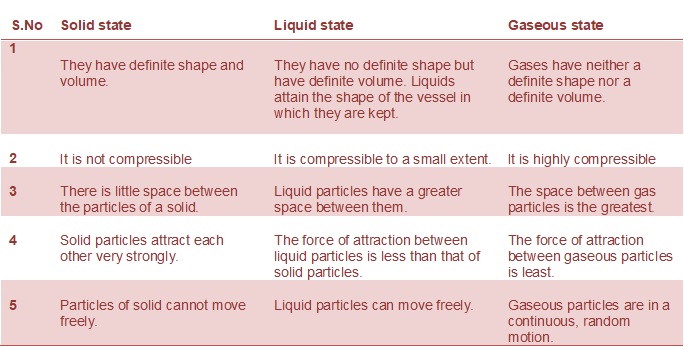
Compressibility is the ability of matter to get reduced to a lower volume when a force or pressure is applied.
Fluidity is the ability of a substance to flow.
By filling a gas container we mean the attainment of the shape of the container by gas.
Shape is defined a definite boundary or physical appearance of something.
Kinetic energy is the energy possessed by a body due to its motion.
Density is the mass per unit volume.
Question 3
Give reasons
Answer 3
Question 4
Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Answer 4
Density is mass per unit volume of a substance (density = mass/volume).
The density of a substance decreases as its volume increases.
Ice has large number of empty spaces between its particles and these spaces are larger as compared to the spaces present between the particles of water. Thus, the volume of ice is greater than that of water and therefore the density of ice is less than that of water. A substance with lower density than water can float on water and that is why, ice floats on water.
Yearlong program for Olympiads preparation & to build necessary skills for future.
Explore More
Time to mark your calendar with the upcoming Olympiads exam schedule.
Explore More
Take your Olympiad preparation to next-level by taking LIVE Classes.
Explore More
Assess your performance by taking topic-wise and full length mock tests.
Explore More
Online tuitions for international compeitions like SASMO, SEAMO, etc for Grades 1-11.
Explore More