Science
(www.olympiadsuccess.com)
Chapter 1: Matter in Our Surroundings
Class: IX
Exemplar sheet 2
Multiple Choice Questions
Question 1
Which one of the following sets of phenomena would increase on raising the temperature?
(a) Diffusion, evaporation, compression of gases
(b) Evaporation, compression of gases, solubility
(c) Evaporation, diffusion, expansion of gases
(d) Evaporation, solubility, diffusion, compression of gases
Answer 1
(c)
Question 2
Seema visited a Natural Gas Compressing Unit and found that the gas can be liquefied under
specific conditions of temperature and pressure. While sharing her experience with friends
she got confused. Help her to identify the correct set of conditions
(a) Low temperature, low pressure
(b) High temperature, low pressure
(c) Low temperature, high pressure
(d) High temperature, high pressure
Answer 2
(c)
Question 3
The property to flow is unique to fluids. Which one of the following statements is correct?
(a) Only gases behave like fluids
(b) Gases and solids behave like fluids
(c) Gases and liquids behave like fluids
(d) Only liquids are fluids
Answer 3
(c)
Question 4
During summer, water kept in an earthen pot becomes cool because of the phenomenon of
(a) diffusion
(b) transpiration
(c) osmosis
(d) evaporation
Answer 4
(d)
Question 5
A few substances are arranged in the increasing order of ‘forces of attraction’ between their
particles. Which one of the following represents a correct arrangement?
(a) Water, air, wind
(b) Air, sugar, oil
(c) Oxygen, water, sugar
(d) Salt, juice, air
Answer 5
(c)
Question 6
On converting 25°C, 38°C and 66°C to kelvin scale, the correct sequence of temperature will be
(a) 298 K, 311 K and 339 K
(b) 298 K, 300 K and 338 K
(c) 273 K, 278 K and 543 K
(d) 298 K, 310 K and 338 K
Answer 6
(a)
Question 7
Choose the correct statement of the following
(a) conversion of solid into vapours without passing through the liquid state is called
vapourisation.
(b) conversion of vapours into solid without passing through the liquid state is called
sublimation.
(c) conversion of vapours into solid without passing through the liquid state is called freezing.
(d) conversion of solid into liquid is called sublimation.
Answer 7
(b)
Question 8
The boiling points of diethyl ether, acetone and n-butyl alcohol are 35°C, 56°C and 118°C
respectively. Which one of the following correctly represents their boiling points in kelvin
scale?
(a) 306 K, 329 K, 391 K
(b) 308 K, 329 K, 392 K
(c) 308 K, 329 K, 391 K
(d) 329 K, 392 K, 308 K
Answer 8
(c)
Question 9
Which condition out of the following will increase the evaporation of water?
(a) Increase in temperature of water
(b) Decrease in temperature of water
(c) Less exposed surface area of water
(d) Adding common salt to water
Answer 9
(a)
Question 10
In which of the following conditions, the distance between the molecules of hydrogen gas
would increase?
(i) Increasing pressure on hydrogen contained in a closed container
(ii) Some hydrogen gas leaking out of the container
(iii) Increasing the volume of the container of hydrogen gas
(iv) Adding more hydrogen gas to the container without increasing the volume of the container
(a) (i) and (iii)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer 10
(c)
Short Answer Questions
Question 11
A sample of water under study was found to boil at 102°C at normal temperature and
pressure. Is the water pure? Will this water freeze at 0°C? Comment.
Answer 11
No the water is not pure and It’s freezing point will be below 0°C due to the presence of a non-volatile impurity in it.
Question 12
A student heats a beaker containing ice and water. He measures the temperature of the
content of the beaker as a function of time. Which of the following (Fig. 1.1) would correctly
represent the result? Justify your choice.
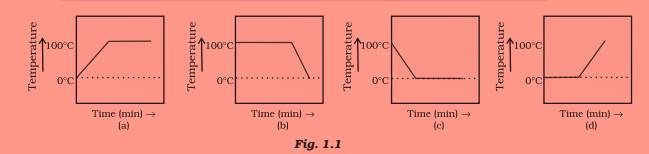
Answer 12
The correct option is (d).
Since ice and water are in equilibrium, the temperature would be zero. When we heat the mixture, the energy supplied is utilized for melt the ice and the temperature does not change till all the ice melts because of latent heat of fusion. On further heating, the temperature of the water would increase.
Question 13
Fill in the blanks:
(a) Evaporation of a liquid at room temperature leads to a——— effect.
(b) At room temperature the forces of attraction between the particles of solid substances are—
——than those which exist in the gaseous state.
(c) The arrangement of particles is less ordered in the ——— state. However, there is no order
in the ——— state.
(d) ——— is the change of gaseous state directly to solid state without going through the ———
state.
(e) The phenomenon of change of a liquid into the gaseous state at any temperature below its
boiling point is called———.
Answer 13
(a) cooling
(b) stronger
(c) liquid, gaseous
(d) sublimation, liquid
(e) evaporation
Question 14
Match the physical quantities given in column A to their S I units given in column B :
(A) (B)
(a) Pressure (i) cubic metre
(b) Temperature (ii) kilogram
(c) Density (iii) pascal
(d) Mass (iv) kelvin
(e) Volume (v) kilogram per cubic metre
Answer 14
(a) — (iii)
(b) — (iv)
(c) — (v)
(d) — (ii)
(e) — (i)
Question 15
The non S I and S I units of some physical quantities are given in column A and column B
respectively. Match the units belonging to the same physical quantity:
(A) (B)
(a) degree celsius (i) kilogram
(b) centimetre (ii) pascal
(c) gram per centimetre cube (iii) metre
(d) bar (iv) kelvin
(e) milligram (v) kilogram per metre cube
Answer 15:
(a) — (iv)
(b) — (iii)
(c) — (v)
(d) — (ii)
(e) — (i)
Question 16:
‘Osmosis is a special kind of diffusion’. Comment.
Answer 16:
Yes, this is true. In both diffusion and osmosis, there is a movement of particles from higher concentrated region to that of lower concentrated region. However, in the case of osmosis the movement of solvent is through a semi permeable membrane which is permeable only to water molecules.
Question 17:
Classify the following into osmosis/diffusion
(a) Swelling up of a raisin on keeping in water.
(b) Spreading of virus on sneezing.
(c) Earthworm dying on coming in contact with common salt.
(d) Shrinking of grapes kept in thick sugar syrup.
(e) Preserving pickles in salt.
(f) Spreading of smell of cake being baked through out the house.
(g) Aquatic animals using oxygen dissolved in water during respiration.
Answer 17:
(a) Osmosis
(b) Diffusion
(c) Osmosis
(d) Osmosis
(e) Osmosis
(f) Diffusion
(g) Diffusion
Question 18:
Water as ice has a cooling effect, whereas water as steam may cause severe burns. Explain
these observations.
Answer 18:
In case of ice the water molecules have low energy while in the case of steam the water molecules have high energy. The high energy of water molecules in steam is transformed as heat and may cause burns. And, in the case of ice, water molecules take energy from the body and thus give a cooling effect.
Question 19:
Alka was making tea in a kettle. Suddenly she felt intense heat from the puff of steam gushing out of the spout of the kettle. She wondered whether the temperature of the steam was higher than that of the water boiling in the kettle. Comment.
Answer 19:
Both the boiling water and steam has a temperature of 100°C, but the steam has more energy because of latent heat of vapourisation.
Question 20:
A glass tumbler containing hot water is kept in the freezer compartment of a refrigerator
(temperature < 0°C). If you could measure the temperature of the content of the tumbler,
which of the following graphs (Fig.1.2) would correctly represent the change in its
temperature as a function of time.
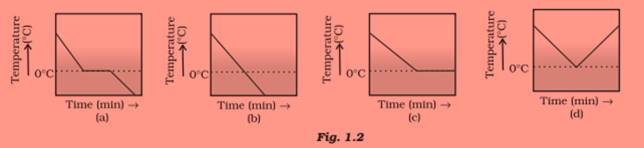
Answer 20:
Graph (a). Initially the water will cool till it reaches the freezing point (0o C). Till all the water will freeze, the temperature will remain constant in this stage and after this temperature would fall again.
Question 21:
Look at Fig. 1.3 and suggest in which of the vessels A,B, C or D the rate of evaporation will
be the highest? Explain.
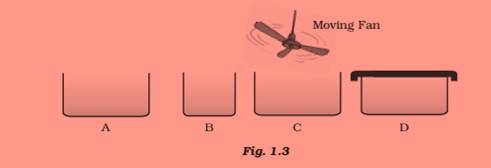
Answer 21:
Vessel C. This is because, evaporation is a surface phenomenon, so the rate of evaporation increases with an increase of surface area . Also, with the increase in air speed, the particles of water vapour will move away with the air, which will increase the rate of evaporation.
Question 22:
(a) Conversion of solid to vapour is called sublimation. Name the term used to denote the
conversion of vapour to solid.
(b) Conversion of solid state to liquid state is called fusion; what is meant by latent heat of
fusion?
Answer 22:
(a) Sublimation
(b) The amount of heat required to convert 1 kilogram of solid into liquid at one atmosphere pressure at its melting point is known as its latent heat of fusion.
Long Answer Questions
Question 23:
You are provided with a mixture of naphthalene and ammonium chloride by your teacher.
Suggest an activity to separate them with well labeled diagram.
Answer 23:
Hint— Naphthalene is insoluble in water but soluble in ether an organic solvent. It is volatile at room temperature. Ammonium chloride is soluble in water and volatile at higher temperature. It decomposes on heating to dryness.
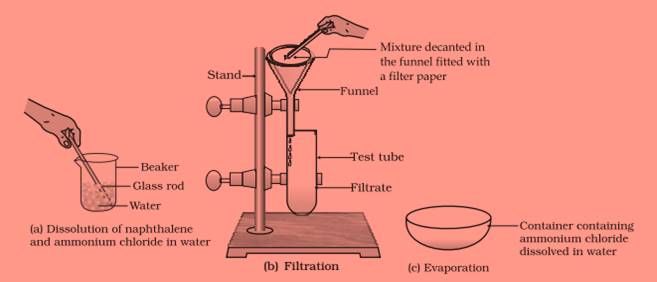
Question 24:
It is a hot summer day, Priyanshi and Ali are wearing cotton and nylon clothes respectively.
Who do you think would be more comfortable and why?
Answer 24:
Cotton is a better absorber of water than the nylon. So cotton helps in absorption of sweat followed by evaporation which leads to cooling. So Priyanshi is more comfortable, whereas Ali is not so comfortable.
Question 25:
You want to wear your favourite shirt to a party, but the problem is that it is still wet after a
wash. What steps would you take to dry it faster?
Answer 25:
Conditions that can increase the rate of evaporation of water are
(a) an increase of surface area by spreading the shirt
(b) an increase in temperature by putting the shirt under the sun
(c) increase the wind speed by spreading it under the fan.
Question 26:
Comment on the following statements:
(a) Evaporation produces cooling.
(b) Rate of evaporation of an aqueous solution decreases with increase in humidity.
(c) Sponge though compressible is a solid.
Answer 26:
(a) Evaporation produce cooling this is because the particles at the liquid surface gain energy from the surroundings and change into vapour thereby producing a cooling effect.
(b) Air around us cannot hold more than a definite amount of water vapour at a given temperature. So, rate of evaporation of water will decrease, this is because if the air is already rich in water vapour, it will not take up more water therefore,.
(c) A sponge has minute holes in which air is trapped. Also the material is not rigid. When we press it, the air is expelled out and we are able to compress it.
Question 27
Why does the temperature of a substance remain constant during its melting point or boiling
point?
Answer 27
Untill all the substance melts or boils, the temperature of a substance remains constant at its melting and boiling points because, the heat supplied is continuously used up in changing the state of the substance by overcoming the forces of attraction between the particles. This heat energy absorbed without showing any rise in temperature is latent heat of fusion/latent heat of vapourisation.
Yearlong program for Olympiads preparation & to build necessary skills for future.
Explore More
Time to mark your calendar with the upcoming Olympiads exam schedule.
Explore More
Take your Olympiad preparation to next-level by taking LIVE Classes.
Explore More
Assess your performance by taking topic-wise and full length mock tests.
Explore More
Online tuitions for international compeitions like SASMO, SEAMO, etc for Grades 1-11.
Explore More