Science
(www.olympiadsuccess.com)
Chapter 14: Chemical Effects of Electric Current
Class: VIII
NCERT Solutions
Question 1
Fill in the blanks
(a) Most liquids that conduct electricity are solutions of _________, __________ and ________.
(b) The passage of an electric current through a solution causes ____________ effects.
(c) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the ____________ terminal of the battery.
(d) The process of depositing a layer of any desired metal on another material by means of electricity is called ____________.
Answer 1
Fill in the blanks
(a) Most liquids that conduct electricity are solutions of acids, bases and salts.
(b) The passage of an electric current through a solution causes chemical effects.
(c) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the negative terminal of the battery.
(d) The process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
Question 2
When the free ends of a tester are dipped into a solution, the magnetic needle shows deflection. Can you explain the reason?
Answer 2
The deflection of magnetic needle indicates electric current is flowing through the wire. It means the liquid or the solution is a good conductor of electricity. When the free ends of a tester are dipped into a solution, electric circuit is completed and an electric current passes through the solution.
Question 3
Name three liquids, which when tested in the manner shown in Fig.14.9, may cause the magnetic needle to deflect.
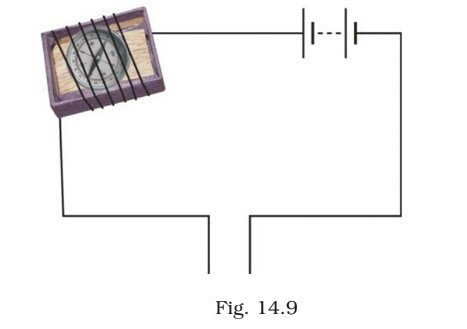
Answer 3
Ground water, vinegar, citric fruit juice. The liquid solutions containing salts (basic or acidic) will conduct electricity.
Question 4
The bulb does not glow in the setup shown in Fig.14.10. List the possible reasons. Explain your answer.
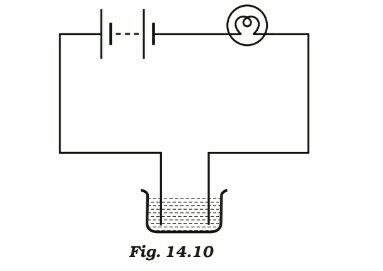
Answer 4
There may be the following possible reasons for not to glow:
Question 5
A tester is used to check the conduction of electricity through two liquids, labelled A and B. It is found that the bulb of the tester glows brightly for liquid A while it glows very dimly for liquid B. You would conclude that
Answer 5
Question 6
Does pure water conduct electricity? If not, what can we do to make it conducting?
Answer 6
Pure or distilled water does not contain salts. Therefore it is a poor conductor of electricity. We can add impurities like salt, lemon juice, vinegar etc. to make it conducting.
Question 7
In case of a fire, before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Answer 7
The water usually contain salts and is a good conductor of electricity. To save themselves and others from electric shock and to avoid any short circuit, firemen shut off the main electrical supply for the area.
Question 8
A child staying in a coastal region tests the drinking water and also the seawater with his tester. He finds that the compass needle deflects more in the case of seawater. Can you explain the reason?
Answer 8
Drinking water is chemically treated and purified by removing various impurities and salts from it. While the sea water contains lots of mineral salts. Therefore, sea water produce more ions (more electric charges) as compared to drinking water and the child sees more deflection in magnetic needle in case of sea water.
Question 9
Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
Answer 9
No it is very risky and unsafe to carry out electric repairs outdoors during heavy downpour. Water (when impure)is a good conductor of electricity and there are chances of getting electric shock.
Question 10
Paheli had heard that rainwater is as good as distilled water. So she collected some rainwater in a clean glass tumbler and tested it using a tester. To her surprise she found that the compass needle showed deflection. What could be the reasons?
Answer 10
Air in our surroundings contains gasses, suspended dust particles and pollutants. These particles gets dissolved in rain water and make it good conducting medium of electricity.
Question 11
Prepare a list of objects around you that are electroplated.
Answer 11
Cold drink cans are tin plated. Artificial Jewellery items are silver or gold-plated. Car bumpers and cycle handles are chromium plated. Metal doors, door handles are zinc plated.
Question 12
The process that you saw in Activity 14.7 is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of battery and why?
Answer 12
Impure Copper plate should be connected to positive terminal. Pure copper plate should be connected to negative terminal of electrode as copper ions are positively charged and will attract to negative electrode terminal.
Yearlong program for Olympiads preparation & to build necessary skills for future.
Explore More
Time to mark your calendar with the upcoming Olympiads exam schedule.
Explore More
Take your Olympiad preparation to next-level by taking LIVE Classes.
Explore More
Assess your performance by taking topic-wise and full length mock tests.
Explore More
Online tuitions for international compeitions like SASMO, SEAMO, etc for Grades 1-11.
Explore More